One of the most severe and merciless illnesses – Huntington’s – has been effectively managed for the first time, according to medical professionals.
The illness spreads among family members, constantly destroys brain cells, and is similar to a mix of dementia, Parkinson’s, and motor neuron disease.
The innovative therapy involves gene treatment administered over a period of 12 to 18 hours during intricate brain surgery.
A heartfelt research group became emotional while explaining that data indicates the disease progressed 75% slower in patients.
This implies that the drop typically seen in a single year would span four years following treatment, allowing patients to enjoy several decades of “high quality life,” said Prof Sarah Tabrizi to News.
The initial signs of Huntington’s disease usually emerge during your 30s or 40s and typically lead to death within two decades – suggesting that early intervention might stop symptoms from ever developing.
Professor Tabrizi, head of the University College London Huntington’s Disease Centre, called the findings “remarkable”.
“We never could have imagined a 75% reduction in the advancement of the disease,” she stated.
None of the treated patients are being disclosed, but one has been medically discharged and has resumed employment. Other participants in the study continue to walk, even though they were anticipated to require a wheelchair.
The treatment is expected to be highly costly. Nevertheless, this marks a time of genuine optimism for a disease that affects individuals in their prime and deeply impacts families.

Huntington’s disease is present in the family of Jack May-Davis. He carries the defective gene responsible for the condition, as did his father, Fred, and his grandmother, Joyce.
Jack described it as “truly terrible and dreadful” to witness his father’s inevitable deterioration.
The initial signs emerged in Fred’s late 30s, such as alterations in his behavior and movement. He ultimately required round-the-clock palliative care until his passing at the age of 54 in 2016.
Jack is 30 years old, works as a clerk for a barrister, is recently engaged to Chloe, and has been involved in research at UCL to transform his diagnosis into something beneficial.
But he had always been aware that he was meant to follow his father’s path, until this day.
He now states that the “truly amazing” advancement has left him “overwhelmed,” enabling him to envision a future that “seems just a little brighter, it does let me consider that my life might be that much longer.”

Huntington’s disease results from a mutation in a segment of our DNA known as the huntingtin gene.
This change transforms a regular protein essential for brain function – known as the huntingtin protein – into a destroyer of nerve cells.
The objective of the therapy is to permanently lower the amount of this harmful protein with one single dose.
The treatment employs advanced genetic medicine that integrates gene therapy and gene suppression techniques.
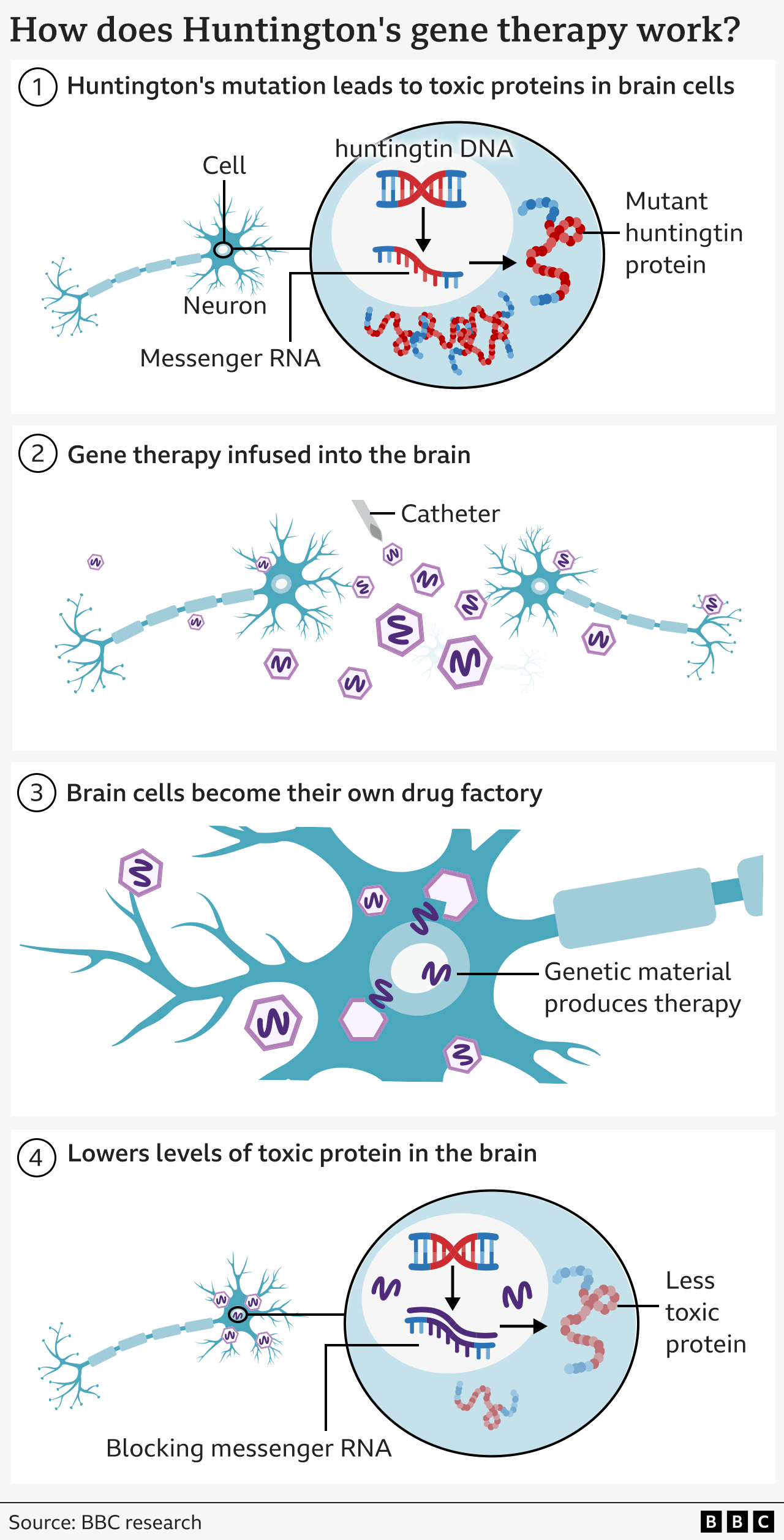
It begins with a harmless virus that has been modified to include a uniquely engineered DNA sequence.
This is deeply integrated into the brain with the help of real-time MRI imaging to direct a microcatheter to two areas of the brain – the caudate nucleus and the putamen. The procedure lasts between 12 and 18 hours of neurosurgical intervention.
The virus functions as a tiny messenger – transporting the new DNA segment into brain cells, where it becomes active.
This transforms the neurons into a production site for the treatment that prevents their own demise.
Cells generate a tiny portion of genetic material (known as microRNA) intended to block and neutralize the messages (referred to as messenger RNA) that are transmitted from the cells’ DNA for creating mutant huntingtin.
This leads to reduced amounts of mutant huntingtin in the brain.
Findings from the study – which included 29 participants – have been shared in a statement from the company uniQure, but have not yet been fully published for evaluation by other experts.
The findings indicated that three years post-surgery, the disease progressed at an average rate of 75% slower, as measured by an assessment that integrates cognitive performance, motor skills, and the capacity to handle everyday tasks.
The data also indicates that the treatment is preserving brain cells. Neurofilament levels in spinal fluid—a clear indicator of brain cell death—were expected to rise by a third if the disease kept advancing, but instead were lower than the initial levels of the trial.
“This is the outcome we have been anticipating,” stated Prof Ed Wild, a consultant neurologist at the National Hospital for Neurology and Neurosurgery affiliated with UCLH.
It was highly unlikely we would ever witness something like this, so living in a world where we know it’s not just possible, but the scale of the impact is astonishing, makes it hard to fully express the feelings.
He mentioned he felt “a little emotional” when considering the effect it might have on families.
The therapy was regarded as safe, even though some individuals experienced inflammation due to the virus, leading to headaches and disorientation, which either subsided on their own or required steroid intervention.
Professor Wild expects the treatment “to be lifelong” since brain cells are not replenished by the body in the same way that blood, bone, and skin cells are continuously regenerated.
Around 75,000 individuals in the UK, US, and Europe are affected by Huntington’s disease, while hundreds of thousands are carriers of the gene mutation, which means they will eventually develop the condition.
UniQure plans to submit a license application in the United States during the first quarter of 2026, with the goal of introducing the medication later that year. Discussions with regulators in the UK and Europe are set to begin next year, although the primary emphasis remains on the US market.
Dr. Walid Abi-Saab, the chief medical officer at uniQure, expressed he was “extremely enthusiastic” about the implications of the results for families, and mentioned that the therapy has “the ability to significantly change” Huntington’s disease.
Nevertheless, the medication will not be accessible to all because of the intricate procedure and the expected high price.
“Undoubtedly, it will be costly,” says Professor Wild.
There is no official cost for the medication. Gene treatments tend to be expensive, yet their long-term benefits can make them cost-effective. In the UK, the NHS covers a £2.6 million per patient gene therapy for haemophilia B.
Professor Tabrizi states that this gene treatment “marks the start” and will pave the way for therapies capable of benefiting a larger population.
She honored the “genuinely courageous” volunteers involved in the study, expressing that she was “delighted for the patients and their loved ones.”
She is currently collaborating with a group of young individuals who are aware they carry the gene but have not yet exhibited symptoms—referred to as stage zero Huntington’s—and is working towards conducting the first prevention trial to determine if the disease can be substantially delayed or potentially halted entirely.






Leave a comment